Stabilized chlorine dioxide
- ■Virus I
![]()
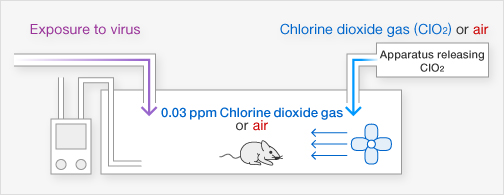
[Method]
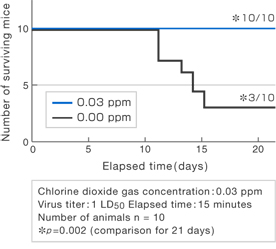
Mortality rates among 10 mice were compared over 21 days when exposed to either chlorine dioxide gas (0.03 ppm) or air for 15 minutes simultaneously with Virus I (1LD50).
〔Results-1〕
In the presence of 0.03 ppm chlorine dioxide gas, the death of mice due to infection with Virus I was prevented (p = 0.002). The level of chlorine dioxide gas to which humans can safely be exposed for long periods of time is 0.1 ppm (time-weighted average [TWA] for human exposure for 8 hours).
〔Results-2〕
In the presence of chlorine dioxide gas (0.03 ppm), Virus I in the lungs of mice was significantly reduced (p = 0.003).
For details, please refer to the abstract of the paper.![]()
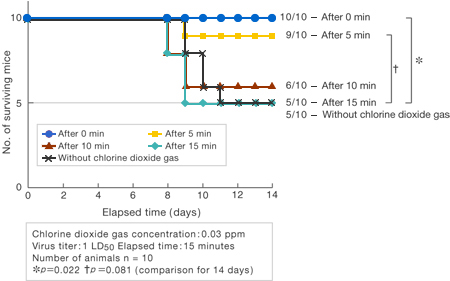
[Method]
The number of deaths among 10 mice was monitored in the case where exposure to chlorine dioxide gas (0.03 ppm) was started either simultaneously with 0, or 5, 10, or 15 minutes after exposure to Virus I (1LD50), and it was compared to the case where the mice were not exposed to the chlorine dioxide gas.
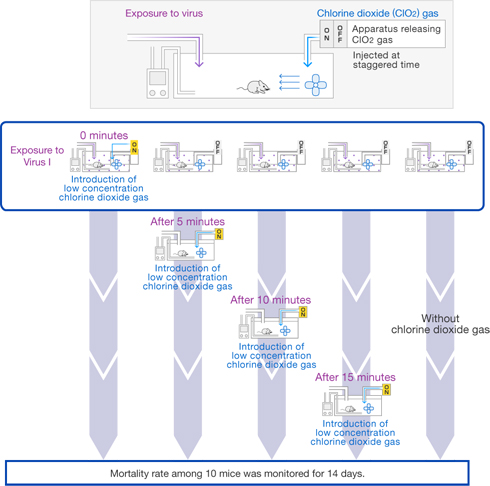
〔Results〕
When mice were exposed to chlorine dioxide gas starting 15 minutes after exposure to Virus I, the same number of mice (5) died as when chlorine dioxide gas was not used, but when the mice were exposed to chlorine dioxide gas simultaneously with exposure to Virus I, none of the mice died at all (p = 0.022).
Moreover, although the difference was not statistically significant (p = 0.081), deaths among the mice were limited to only one animal even when exposure to chlorine dioxide gas was started after the waiting time of 5 minutes.
For details, please refer to the abstract of the paper.![]()
![]()
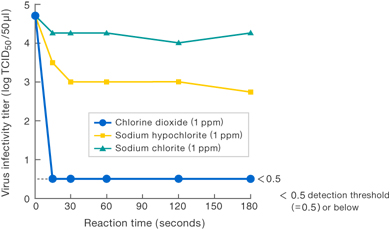
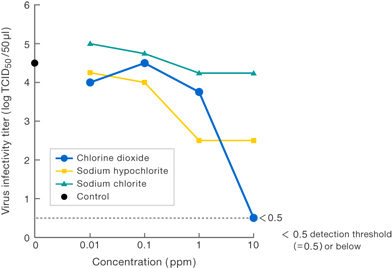
[Method]
Solutions of various substances (each at a concentration of 1 ppm) were allowed to work upon Virus I , and the virus infectivity titer was measured.
〔Results〕
Results suggest that 1 ppm chlorine dioxide suppressed virus infectivity at least 100-fold compared to 1 ppm sodium hypochlorite.
No suppression of infectivity titer was observed for 1 ppm sodium chlorite (commonly known as stabilized chlorine dioxide).
Presented at the 33rd Annual Meeting of Society
for Antibacterial and Antifungal Agents (Tokyo, 2006).
- ■Virus N
![]()
[Method]
In the hermetic space (20℃, 150 L), Virus N suspension (in the moist condition) or dry Virus N (in the dry condition) was placed on the glass petri dish, and after certain hours of exposure to chlorine dioxide (ClO2), viral infectivity titer was determined. The same procedures were followed in the air as the control and the viral infectivity titer was determined. In this experiment, the effect of organic materials was also assessed by the addition of fetal bovine serum (FBS) at a designated concentration.
The relative humidity under the environment was set at the intermediate humidity (45 to 55%) during the exposure to Virus N suspension (in the moist condition), and at the intermediate humidity (45 to 55%) and the high humidity (75 to 85%) during the exposure to dry Virus N (in the dry condition) to assess the effects of humidity.
〔Result -1〕
After 6 hours of exposure of Virus N suspension (in the moist condition) to ClO2 at a low concentration (mean 0.08 ppm) at 20℃ at the intermediate humidity (45 to 55%) without the addition of the organic substance (FBS), the viral infectivity titer decreased by 99.99% or greater compared to the control.
Also, it was revealed that the low-level ClO2’s action to lower the viral infectivity titer was mitigated under the presence of the organic substance (FBS).
〔Result- 2〕
After 24 hours of exposure of dry Virus N (in the dry condition) to ClO2 at a low concentration (about 0.08 ppm) at 20℃ at the high humidity (75 to 85%), the viral infectivity titer decreased by 99.99% or greater. Also, it was revealed that the virus eliminating effect was mitigated under the presence of the organic substance (5% FBS).
At the intermediate humidity (45 to 55%), ClO2 at a low concentration scarcely reduced the viral infectivity titer.
![]()
![]()
[Method]
Solutions with various substances were allowed to work upon Virus N, and the virus infectivity titer was measured.
〔Results-1〕
Results suggest that 4 ppm chlorine dioxide suppressed the virus infectivity at least 100-fold compared to 4 ppm sodium hypochlorite.
No suppression of infectivity titer was observed for sodium chlorite (commonly known as stabilized chlorine dioxide) even at 100 ppm.
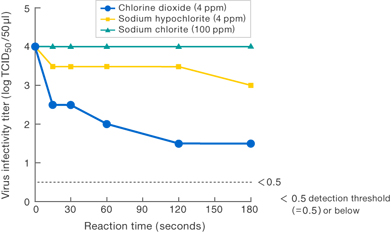
Presented at the 1st Symposium on Pharmaceutical Food Science (Osaka, 2006).
〔Results-2〕
Results suggest that the viral infectivity was suppressed at least 100,000-fold by 10 ppm chlorine dioxide compared to 10 ppm povidone iodine.
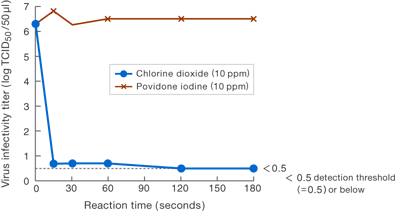
Taiko Pharmaceutical Company’s examination data
- ■Virus E
![]()
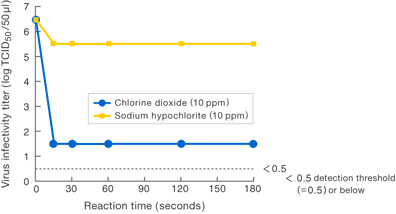
[Method]
Solutions with various substances were allowed to work upon Virus E for 1 minute at specific concentrations, and the virus infectivity titer was measured.
〔Results〕
Results suggest that 10 ppm chlorine dioxide suppressed the virus infectivity at least 100-fold compared to 10 ppm sodium hypochlorite.
No suppression of the infectivity titer was observed for sodium chlorite (commonly known as stabilized chlorine dioxide).
Presented at the 33rd Annual Meeting of Society
for Antibacterial and Antifungal Agents (Tokyo, 2006).
- ■Virus A
![]()
[Method]
Solutions with various substances (each at a concentration of 10 ppm) were allowed to work upon Virus A, and the virus infectivity titer was measured.
〔Results〕
Results suggest that 10 ppm chlorine dioxide suppressed the virus infectivity at least 10,000-fold compared to 10 ppm sodium hypochlorite.
Presented at the 56th Annual Meeting of the Japanese Society
for Virology (Okayama, 2008)
- ■Virus M
[Method]
Solutions with various substances (each at a concentration of 10 ppm) were allowed to work upon Virus M, and the virus infectivity titer was measured.
〔Results〕
Results suggest that 10 ppm chlorine dioxide suppressed the virus infectivity at least 1,000-fold compared to 10 ppm sodium hypochlorite.
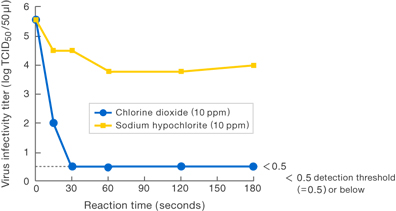
Presented at the 56th Annual Meeting of the Japanese Society
for Virology (Okayama, 2008)
- ■Comparison of the virus eliminating effect of ClO2-dissolved solution and sodium hypochlorite solution
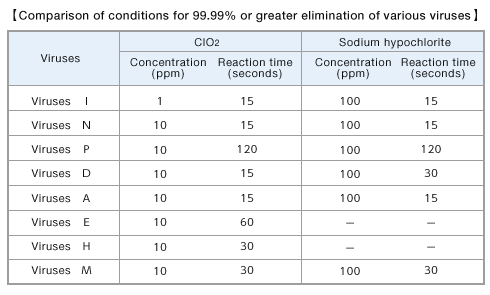
The concentration and period necessary for 99.99% or greater elimination of various viruses were determined after a certain period of exposure of various viruses to ClO2-dissolved solution and sodium hypochlorite solution in the test tube.
Reform from Sanekata T., et al. Biocontrol Science 15(2), 45-49(2010)
Abstract of the paper in Japanese(Anitex)
Abstract of the paper(Biocontrol Science)
- ■References
![]()
Refer to Mimura, T., Fujioka, T., and Mimaru, A.: Effects of the ClO2-releasing agent to prevent influenza-like diseases, Journal of the Japan Society of Environmental Infections : 25(5), 277-280 (2010) for the results of the experiment performed in healthy adults to assess the effects of the ClO2-releasing agent.
Bacteria Validation Data
![]()
[Method]
After a certain days of placement of the ClO2-releasing product in the autopsy room in the hospital, floating microorganisms were captured to the agar medium using the air sampler and the floating microbial count was determined after culturing the agar medium.
〔Result〕
It was revealed that the placement of the ClO2-releasing product reduced the floating microbial count in the room.
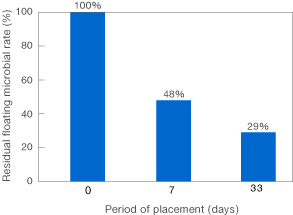
Taiko Pharmaceutical’s test data
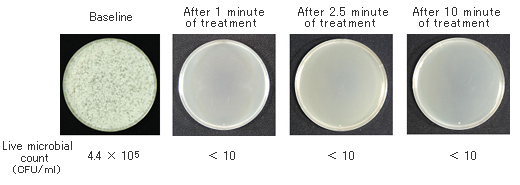
![]()
[Method]
After a certain period of exposure of various bacteria to the test solution at designated concentrations, the residual live bacterial count (CFU/ml) was determined according to the agar plate pour culture method.
〔Result〕
It was suggested that ClO2 more effectively inhibited various microorganism than sodium hypochlorite.
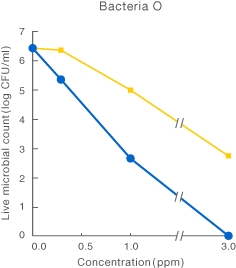
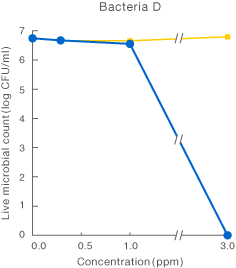
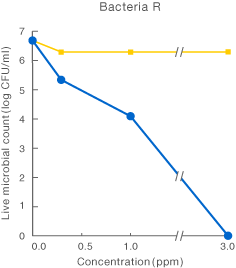
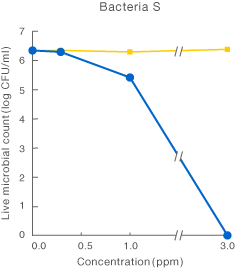
Taiko Pharmaceutical’s test data
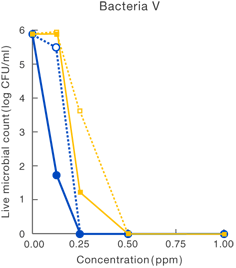
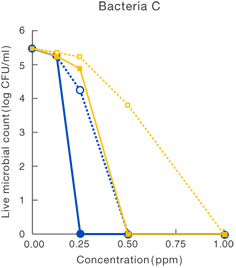

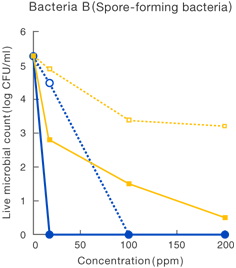
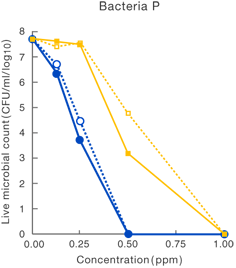


Data released in the 83rd Conference of the Japanese Society
for Bacteriology (Yokohama, 2010)
- ■Summary of ClO2-dissolved solution’s bacteria elimination effect
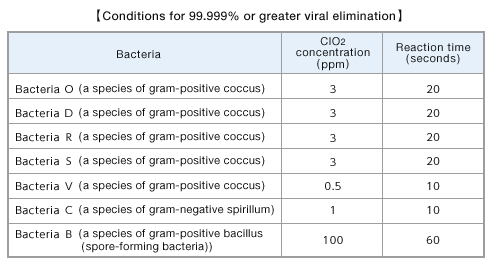
The concentration and period necessary for 99.999% or greater elimination of various bacteria were determined after a certain period of exposure of various viruses to ClO2-dissolved solution in the test tube.
Miura, T. and Shibata, T.: Anitex 21(6), 11-16(2009).
Fungus Validation Data
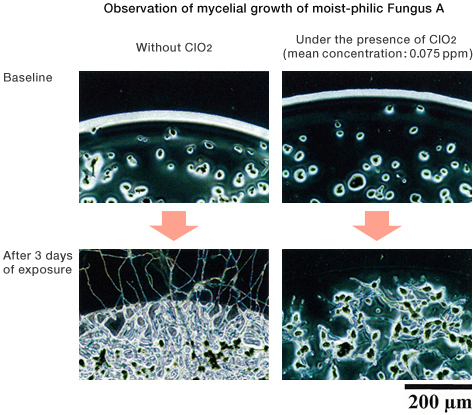
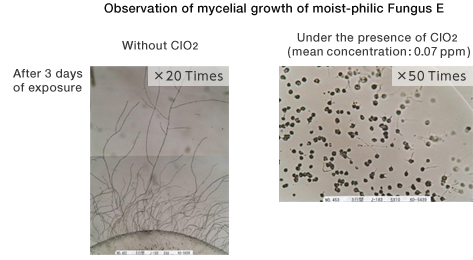
![]()
[Method]
A portable fungal sensor was placed under the air environment or the low-level ClO2 environment at 25℃, and after 3 days of exposure, phase contrast microscopy was performed.
〔Result〕
Under the low-level ClO2 environment, mycelial growth was inhibited in both moist-philic Fungus A and moist-phobic Fungus E.
![]()
Abstract of the paper in Japanese
Morino, H. and Shibata, T.: Clinical Allergy 30(1), 51-55(2010).
![]()
[Method]
ClO2-dissolved solution (ClO2 concentration: 100 ppm) was added to the suspension of Fungus T (filamentous bacterium) and neutralized after a designated hours of reaction at room temperature. The neutralized test solution was inoculated to the agar medium and cultured (25℃±1℃, 7 days).
〔Result〕
No growth of Fungus T (filamentous bacterium) was observed when it was treated with ClO2-dissolved solution (ClO2 concentration: 100 ppm).
It was suggested that treatment with ClO2 at the concentration of 100 ppm eliminated Fungus T (filamentous bacterium) for a short period.
Date of issuance of the certificate of analysis: September 29, 2008
Issuance No. of the certificate of analysis: 208071846-00 (Excerpt)
- ■Summary of ClO2-dissolved solution’s effects to eliminate various fungi
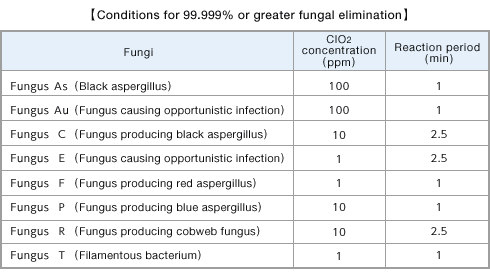
The concentration and period necessary for 99.999% or greater elimination of various fungi were determined after a certain period of exposure of various fungi to ClO2-dissolved solution in the test tube.
Miura, T. and Shibata, T.: Anitex 21(6), 11-16(2009).
Allergic substances test data
![]()
[Method]
Purified antigen solutions of Der f 2 and Cry j l, antigens of mite and pollen origin, respectively, were lyophilized (antigen concentration: 100 ng), and after 24 hours of reaction with the low-concentration ClO2 gas (mean concentration: 0.09 ppm) at 25℃ at a certain relative humidity, each antigen level was determined according to enzyme-linked immunosorbent assay method. The antigen level (%) after the exposure to low-concentration ClO2 gas was obtained when the antigen level under the air environment (control) were designated to be 100%.
〔Result〕
The antigenicity decreased by 60% or greater after 24 hours of exposure of purified antigens of mite and pollen (Der f 2 and Cry j l, respectively) to the low-concentration ClO2 gas (mean concentration: 0.09 ppm).
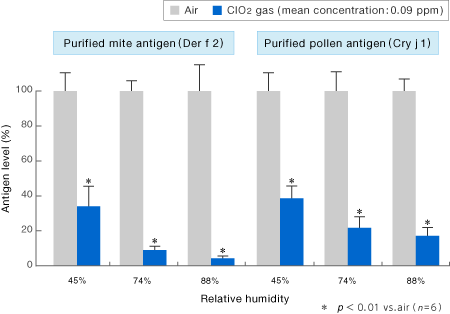
Morino, H. and Shibata, T.: Clinical Allergy 30(1), 51-55(2010).
![]()
[Method]
After 10 minutes of reaction of purified antigens of fungus, mite, and pollen origin (Alt a 1, Der f 2 and Cry j l, respectively) (each concentration: 5 μg/ml) with ClO2 gas-dissolved solution or tap water (free residual chlorine: 0.4 to 0.6 mg/l), antigenicity was determined according to enzyme-linked immunosorbent assay method. The antigen levels (%) after the reaction with ClO2 and tap water were obtained when the antigen level obtained from distilled water (control) were designated to be 100%.
〔Result〕
The antigenicity decreased significantly after 10 minutes of reaction of purified antigens of fungus, mite, and cedar pollen origin (Alt a 1, Der f 2 and Cry j l, respectively) with ClO2 gas-dissolved solution (0.5 to 1 mg/l).
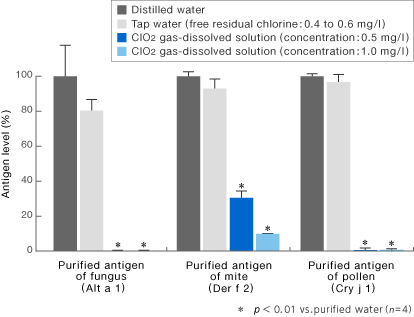
Morino, H. and Shibata, T.: Clinical Allergy 30(1), 51-55(2010).











